Easy Mineral Resource is There Lead Mining in Minnesota
6.5.1: Mineral Resources
- Page ID
- 69394
![By James St. John [<a data-cke-saved-href="http://creativecommons.org/licenses/by/2.0" href="http://creativecommons.org/licenses/by/2.0">CC BY 2.0</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AMother_Lode_Gold_OreHarvard_mine_quartz-gold_vein.jpg" href="https://commons.wikimedia.org/wiki/File%3AMother_Lode_Gold_OreHarvard_mine_quartz-gold_vein.jpg">via Wikimedia Commons</a> The yellow gold is inside white quartz.](https://bio.libretexts.org/@api/deki/files/57537/Mother_Lode_Gold_OreHarvard_mine_quartz-gold_vein-300x209.jpg?revision=1&size=bestfit&width=338&height=235)
Mineral resources, while principally nonrenewable, are generally placed in two main categories: metallic (containing metals) or nonmetallic (containing other useful materials). Metallic minerals are those from which valuable metals (e.g. iron, copper) can be extracted for commercial use. Metals that are considered geochemically abundant occur at crustal abundances of 0.1 percent or more (e.g. iron, aluminum, manganese, magnesium, titanium). Metals that are considered geochemically scarce occur at crustal abundances of less than 0.1 percent (e.g. nickel, copper, zinc, platinum metals). Some important metallic minerals are: hematite (a source of iron), bauxite (a source of aluminum), sphalerite (a source of zinc) and galena (a source of lead). Metallic minerals occasionally but rarely occur as a single element (e.g. native gold or copper). Most mining is focused on metallic minerals. A significant part of the advancement of human society has been developing the knowledge and technologies that yielded metal from the Earth and allowed the machines, buildings, and monetary systems that dominate our world today. The location and recovery of these metals have been a key facet of the study of geology since its inception.
While receiving much less attention, nonmetallic mineral resources (also known as industrial minerals) are just as vital to ancient and modern society as metallic minerals. Nonmetallic minerals are valuable, not for the metals they contain, but for their properties as chemical compounds. Because they are commonly used in industry, they are also often referred to as industrial minerals. They are classified according to their use. The most basic of these is building stone. Limestone, travertine, granite, slate, and marble are common building stones and have been quarried for centuries (Figure \(\PageIndex{2}\)). Some industrial minerals are also used for building materials (e.g. gypsum for plaster and kaolin for bricks). Some industrial minerals are used as sources of important chemicals (e.g. halite for sodium chloride and borax for borates). For everything made out of concrete or asphalt, we need sand and gravel. To make the cement that holds concrete together, we also need limestone. Others are used for making fertilizers (e.g. apatite for phosphate and sylvite for potassium). Still others are used as abrasives (e.g. diamond and corrundum). For the glass in our computer screens and for glass-sided buildings, we need silica sand plus sodium oxide (Na2O), sodium carbonate (Na2CO3), and calcium oxide (CaO). For a wide range of applications (e.g., ceramics and many industrial processes), we also need various types of clay. Some nonmetallic mineral resources are not mineral specific; nearly any rock or mineral can be used. This is generally called aggregate and is used in concrete, roads, and foundations. Gravel is one of the more common aggregates. Quarried rock is also used in some applications where rounded gravel isn't suitable, such as the ballast (road bed) for railways, where crushed angular rock is needed.
![By Michele Buzzi, Studio Cicero. [<a data-cke-saved-href="http://www.gnu.org/copyleft/fdl.html" href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>, <a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0/" href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a> or <a data-cke-saved-href="http://creativecommons.org/licenses/by/2.5" href="http://creativecommons.org/licenses/by/2.5">CC BY 2.5</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AMarblequarry.JPG" href="https://commons.wikimedia.org/wiki/File%3AMarblequarry.JPG">via Wikimedia Commons</a> The image shows a hillside with blocks of marble removed.](https://bio.libretexts.org/@api/deki/files/57539/CarraraMarblequarry-225x300.jpg?revision=1)
Mineral Deposits
Minerals are everywhere around us. For example, the ocean is estimated to contain more than 70 million tons of gold. Yet, it would be much too expensive to recover that gold because of its very low concentration in the water. Minerals must be concentrated into deposits to make their collection economically feasible. A mineral deposit containing one or more minerals that can be extracted profitably is called an ore. Many minerals are commonly found together (e.g. quartz and gold; molybdenum, tin and tungsten; copper, lead and zinc; platinum and palladium). Because various geologic processes can create local enrichments of minerals, mineral deposits can be classified according to the concentration process that formed them. The five basic types of mineral deposits are: hydrothermal, magmatic, sedimentary, placer and residual.
If the material can be mined at a profit, the body constitutes an ore deposit. Typically, the term ore is used for only metal-bearing minerals, though the concept of ore as a non-renewable resource can be applied to valuable concentrations of fossil fuels, building stones, and other non-metal deposits, even groundwater. Mineral ores are found in just a relatively few areas, because it takes a special set of circumstances to create them. Therefore, the signs of a mineral deposit are often small and difficult to recognize. Locating deposits requires experience and knowledge. Geologists can search for years before finding an economic mineral deposit. Deposit size, its mineral content, extracting efficiency, processing costs and market value of the processed minerals are all factors that determine if a mineral deposit can be profitably developed. For example, when the market price of copper increased significantly in the 1970s, some marginal or low-grade copper deposits suddenly became profitable ore bodies.
Magmatic Deposits
Magmatic mineral deposits are formed when processes such as partial melting and fractional crystallization occur during the melting and cooling of rocks. Layered intrusion (typically ultramafic to mafic) can be host to deposits that contain copper, nickel, platinum-palladium-rhodium, and chromium. The Stillwater Complex in Montana is an example of an economic layered mafic intrusion [30]. Associated deposit types can contain chromium or titanium-vanadium. The largest magmatic deposits in the world are the chromite deposits in the Bushveld Igneous Complex in South Africa [31] (Figure \(\PageIndex{3}\)). Rocks of the Bushveld Igneous Complex have an areal extent larger than the state of Utah. The chromite occurs in layers, which resemble sedimentary layers, except this occurred within a crystallizing magma chamber.
![By kevinzim / Kevin Walsh [<a data-cke-saved-href="http://creativecommons.org/licenses/by/2.0" href="http://creativecommons.org/licenses/by/2.0">CC BY 2.0</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AChromitite_Bushveld_South_Africa.jpg" href="https://commons.wikimedia.org/wiki/File%3AChromitite_Bushveld_South_Africa.jpg">via Wikimedia Commons</a> The rock has several layers, with the dark layers being the ones with value.](https://bio.libretexts.org/@api/deki/files/57543/LayeredIntrusionChromitite_Bushveld_South_Africa-300x211.jpg?revision=1)
Water and other volatiles that are not incorporated into mineral crystals while a magma crystallizes become concentrated around the margins of these crystallizing magmas. Ions in these hot fluids are very mobile and can form exceptionally large crystals. Once crystallized, masses of these large crystals are called pegmatites that form from the concentration of magma fluids near the end of crystallization when nearly the entire magma body has crystallized (Figure \(\PageIndex{4}\)). In addition to minerals that are predominant in the main igneous mass, such as quartz, feldspar, and mica, pegmatite bodies may also contain very large crystals of unusual minerals that contain rare elements like beryllium, lithium, tantalum, niobium, and tin, as well as native elements like gold [32]. Such pegmatites are ores of these metals.
![By Parent Géry (Own work) [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0" href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a> or <a data-cke-saved-href="http://www.gnu.org/copyleft/fdl.html" href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AElba%C3%AFte_et_mica_(Br%C3%A9sil)_1.JPG" href="https://commons.wikimedia.org/wiki/File%3AElba%C3%AFte_et_mica_(Br%C3%A9sil)_1.JPG">via Wikimedia Commons</a> The rock is mostly green and purple](https://bio.libretexts.org/@api/deki/files/57540/Elbai%25CC%2588te_et_mica_Bre%25CC%2581sil_1-300x199.jpg?revision=1)
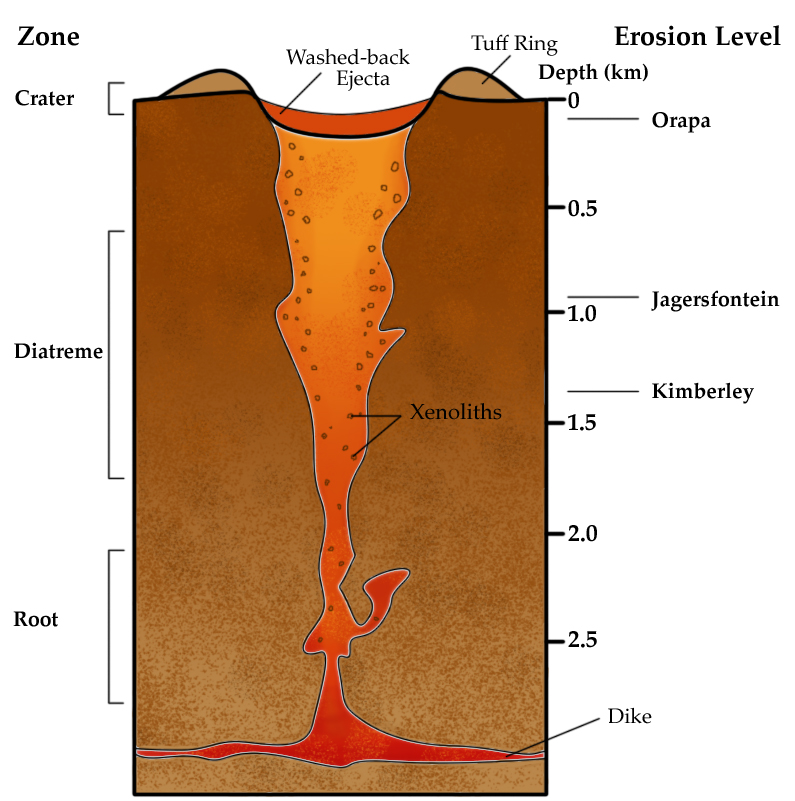
An unusual magmatic process is a kimberlite pipe, which is a volcanic conduit that transports ultramafic magma from depths in the mantle to the surface (Figure \(\PageIndex{5}\)). Diamonds, which are formed at great temperature and depth, are transported this way to locations where they can be mined. The process that emplaced these kimberlite (ultramafic) rocks is no longer common on Earth, and most of the known deposits are Archean [33].
Hydrothermal Deposits
Hydrothermal mineral deposits are formed when minerals are deposited by hot, aqueous solutions flowing through fractures and pore spaces of crustal rock (Figure \(\PageIndex{6}\)). Many famous ore bodies have resulted from hydrothermal deposition, including the tin mines in Cornwall, England and the copper mines in Arizona and Utah.
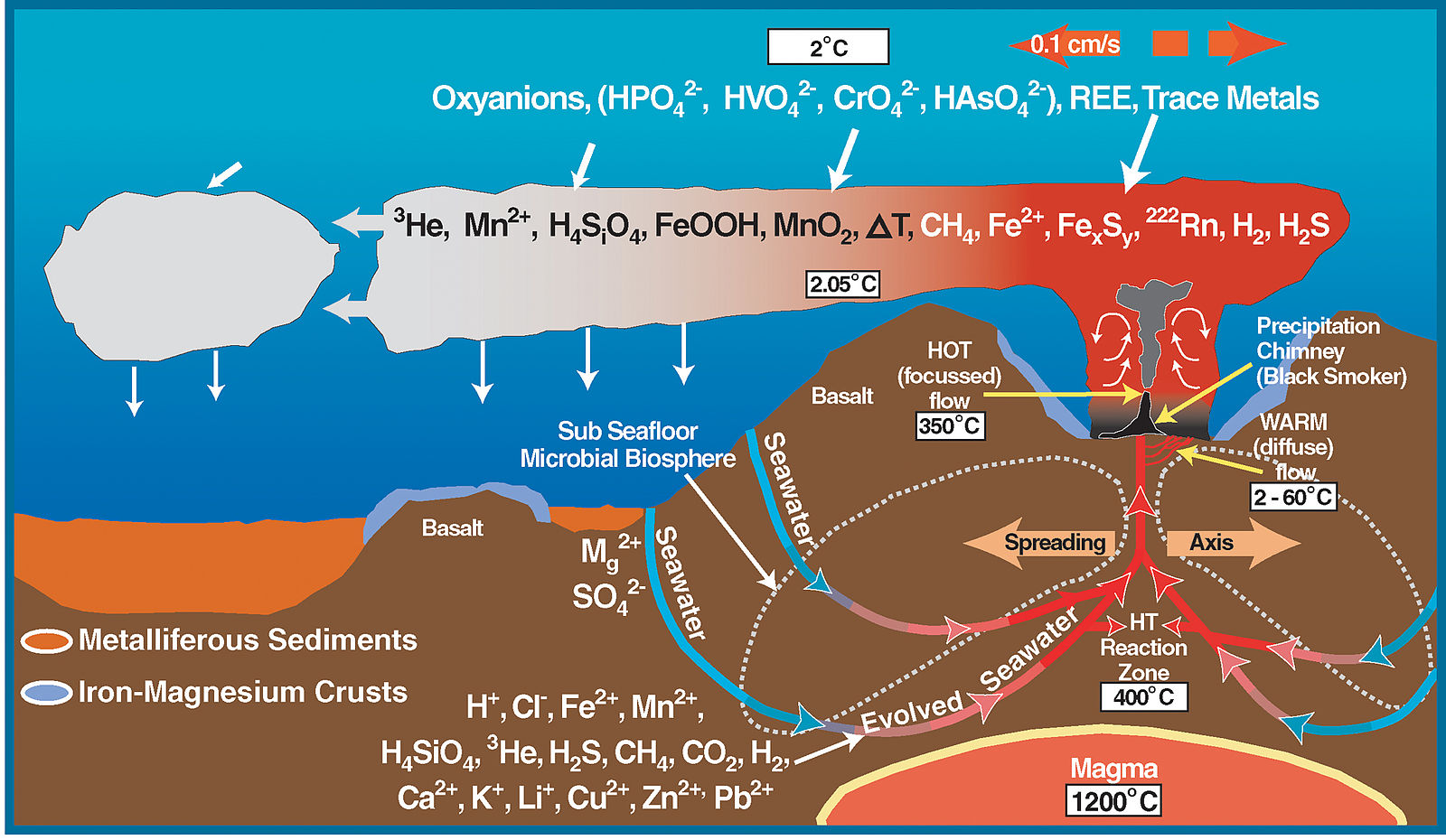
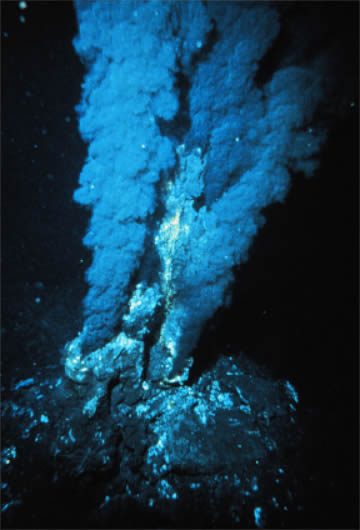
Figure \(\PageIndex{7}\): Black Smoker A billowing discharge of superheated mineral-rich water at an oceanic ridge, in the Atlantic Ocean. Black "smoke" is actually from metallic sulfide minerals that form modern ore deposits. Source: P. Rona of U.S. National Oceanic and Atmospheric Administration via Wikimedia Commons
The most active hydrothermal process today produces volcanogenic massive sulfide (VMS) deposits, which form from black smoker activity (Figure \(\PageIndex{7}\)) near mid-ocean ridges all over the world, and commonly contain copper, zinc, lead, gold, and silver when found on the surface [34]. The largest of these deposits occur in Precambrian age rocks. The Jerome deposit in central Arizona is a good example.
Another type of deposit which draws on heated water from magma is a porphyry deposit (Figure \(\PageIndex{8}\)). This is not to be confused with the igneous texture porphyritic, although the name is derived from the porphyritic texture that is nearly always present in the igneous rocks in a porphyry deposit. Several types of porphyry deposits exist: porphyry copper, porphyry molybdenum, and porphyry tin. They are characterized by the presence of low-grade disseminated ore minerals closely associated with intermediate and felsic intrusive rocks over a very large area [35]. Porphyry deposits are typically the largest mines on Earth. One of the largest, richest, and possibly best-studied mines in the world is Utah's Bingham Canyon open-pit mine, which has had over 100 years of high production of several elements including copper, gold, molybdenum, and silver. Associated underground carbonate replacement deposits have produced lead, zinc, gold, silver, and copper [36]. Past open pit production at this mine was dominated by copper and gold from chalcopyrite and bornite. Gold occurs in minor quantities in the copper-bearing mineral, but the large scale of production makes Bingham Canyon one of the largest gold mines in the U.S. Future production may be more copper and molybdenum (molybdenite) from deeper underground mines.
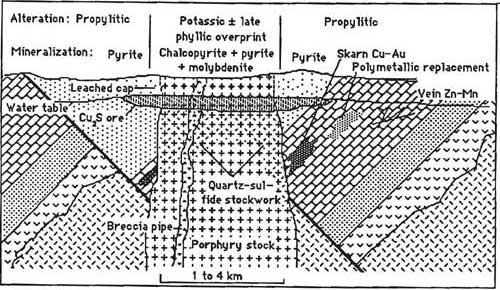
The majority of porphyry copper deposits owe their economic value to concentration by weathering processes occurring millions of years after the hosting intrusion called supergene enrichment (Figure \(\PageIndex{9}\)). These occur once the hydrothermal event has ceased and the ore body has been uplifted, eroded, and exposed to oxidation [37]. When the upper pyrite-rich portion of the deposit is exposed to rain, pyrite in the oxidizing zone creates an extremely acid condition which dissolves copper out of copper minerals such as chalcopyrite, converting the chalcopyrite to iron oxides like hematite or goethite. The copper is carried downward in the solution until it arrives at the groundwater table and a reducing environment where the copper precipitates, converting primary copper minerals into secondary higher-copper content minerals. Chalcopyrite (35% Cu) is converted to bornite (63% Cu) and ultimately chalcocite (80% Cu). Without this enriched zone (2 to 5 times higher in copper content than the main deposit) most porphyry copper deposits would not be economic.
![By Stephanie Salisbury (IMG_4218) [<a data-cke-saved-href="http://creativecommons.org/licenses/by/2.0" href="http://creativecommons.org/licenses/by/2.0">CC BY 2.0</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AMorenci_Mine_2012.jpg" href="https://commons.wikimedia.org/wiki/File%3AMorenci_Mine_2012.jpg">via Wikimedia Commons</a> The mine contains grey rocks, which are not enriched, and red rocks, which is where the enrichment occurs.](https://bio.libretexts.org/@api/deki/files/57541/Morenci_Mine_2012-300x200.jpg?revision=1&size=bestfit&width=365&height=243)
If limestone or other calcareous sedimentary rocks are present adjacent to the magmatic body, then another type of ore deposit called a skarn deposit can form (Figure \(\PageIndex{10}\)). These metamorphic rocks form as magma-derived, highly saline metalliferous fluids react with carbonate rocks, creating calcium-magnesium-silicate minerals like pyroxene, amphibole, and garnet, as well as high-grade zones of iron, copper, and zinc minerals and gold [38]. Intrusions that are genetically related to the intrusion that made the Bingham Canyon deposit have also produced copper-gold skarns that were mined by the early European settlers in Utah [39; 40]. Metamorphism of iron and/or sulfide deposits commonly results in an increase in grain size that makes separation of gangue from the desired sulfide or oxide minerals much easier.
![By Siim Sepp (Sandatlas) (Own work) [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0" href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3A00031_6_cm_grossular_calcite_augite_skarn.jpg" href="https://commons.wikimedia.org/wiki/File%3A00031_6_cm_grossular_calcite_augite_skarn.jpg">via Wikimedia Commons</a> Calcite is blue, augite green, and garnet brown/orange in this rock.](https://bio.libretexts.org/@api/deki/files/57547/16.3_6_cm_grossular_calcite_augite_skarn-300x255.jpg?revision=1)
Sediment-hosted disseminated gold deposits consist of low concentrations of microscopic gold as inclusions and disseminated atoms in pyrite crystals (Figure \(\PageIndex{11}\)). These are formed via low-level hydrothermal reactions (generally in the realm of diagenesis) that occur in certain rock types, namely muddy carbonates and limey mudstones. This hydrothermal alteration is generally far-removed from a magma source but can be found in extended rocks with a high geothermal gradient. The earliest locally mined deposit of this type was the Mercur deposit in the Oquirrh Mountains of Utah where almost one million ounces of gold were recovered between 1890 and 1917. In the 1960s a metallurgical process using cyanide was developed for these types of low-grade ores. These deposits are also called Carlin-type deposits because the disseminated deposit near Carlin, Nevada is where the new technology was first applied and because the first definitive scientific studies were conducted there [41]. Gold was introduced by hydrothermal fluids which reacted with silty calcareous rocks, removing carbonate, creating additional permeability, and adding silica and gold-bearing pyrite in the pore space between grains. The Betze-Post mine and the Gold Quarry mine on the "Carlin Trend" are two of the largest of the disseminated gold deposits in Nevada. Similar deposits, but not as large, have been found in China, Iran, and Macedonia [42].
![<a data-cke-saved-href="https://en.Wikipedia.org/wiki/User:Qfl247" href="https://en.Wikipedia.org/wiki/User:Qfl247" class="extiw" title="en:User:Qfl247">Matt Affolter</a> at <a class="external text" data-cke-saved-href="http://en.Wikipedia.org" href="http://en.Wikipedia.org">en.Wikipedia</a> [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0" href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a> or <a data-cke-saved-href="http://www.gnu.org/copyleft/fdl.html" href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AGoldinPyriteDrainage_acide.JPG" href="https://commons.wikimedia.org/wiki/File%3AGoldinPyriteDrainage_acide.JPG">from Wikimedia Commons</a> The rock is red.](https://bio.libretexts.org/@api/deki/files/57538/GoldinPyrite-300x240.jpg?revision=1&size=bestfit&width=320&height=256)
Deposits from Sedimentary and Weathering Processes
![<a data-cke-saved-href="https://en.Wikipedia.org/wiki/User:Qfl247" href="https://en.Wikipedia.org/wiki/User:Qfl247" class="extiw" title="Wikipedia:User:Qfl247">Matt Affolter</a> at <a data-cke-saved-href="https://en.Wikipedia.org/wiki/" href="https://en.Wikipedia.org/wiki/" class="extiw" title="Wikipedia:">English Wikipedia</a> [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0" href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a> or <a data-cke-saved-href="http://www.gnu.org/copyleft/fdl.html" href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AUraniumMineUtah.JPG" href="https://commons.wikimedia.org/wiki/File%3AUraniumMineUtah.JPG">via Wikimedia Commons</a> A dark shaft runs into the mountain.](https://bio.libretexts.org/@api/deki/files/57535/16.1_UraniumMineUtah-300x225.jpg?revision=1&size=bestfit&width=325&height=244)
Geochemical processes that occur at or near the surface without the aid of magma also concentrate metals, but to a lesser degree than hydrothermal processes. One of the main reactions is redox (short for reduction/oxidation) chemistry, which has to do with the amount of available oxygen in a system. Places where oxygen is plentiful, as in the atmosphere today, are considered oxidizing environments, while oxygen-poor environments are considered reducing. Uranium deposition is an example of redox mobilization. Uranium is soluble in oxidizing groundwater environments and precipitates as uraninite when reducing conditions are encountered. Many of the deposits across the Colorado Plateau (e.g. Moab, Utah) were formed by this method [43].
Redox reactions were also responsible for the creation of banded iron formations (BIFs), which are interbedded layers of iron oxide (hematite and magnetite), chert, and shale beds (Figure \(\PageIndex{13}\)). These deposits formed early in the Earth's history as the atmosphere was becoming oxygenated. Cyclic oxygenation of iron-rich waters initiated the precipitation of the iron beds. Because BIFs are generally Precambrian in age, they are only found in some of the older exposed rocks in the United States, in the upper peninsula of Michigan and northeastern Minnesota [44].

Figure \(\PageIndex{13}\): Banded iron formation from an unknown location in North America on display at a museum in Germany. The rock is about 2 m across. The dark grey layers are magnetite and the red layers are hematite. Chert is also present. [https://upload.wikimedia.org/wikiped...%28aka%29.jpg]
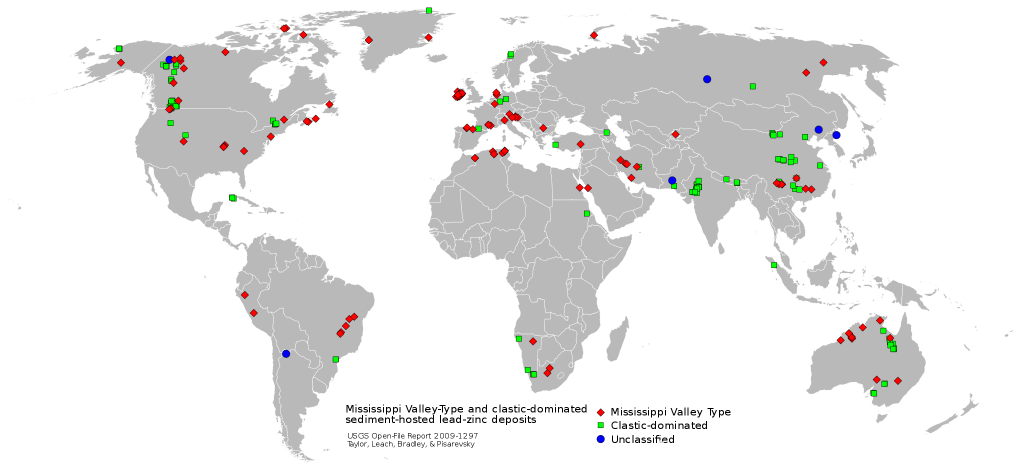
Deep, saline, connate fluids (trapped in the pore spaces), within sedimentary basins may be highly metalliferous. When expelled outward and upward during basin compaction, these fluids may form lead and zinc deposits in limestone by replacement or by filling open spaces (caves, faults) and in sandstone by filling pore spaces. The most famous of these are called Mississippi Valley-type deposits [44] (Figure \(\PageIndex{14}\)). Also known as carbonate-hosted replacement deposits, they are large deposits of galena and sphalerite (lead and zinc ores) which form from fluids in the temperature range of 100 to 200°C. Although they are named for occurrences along the Mississippi River Valley in the United States, they are found worldwide.
Sediment-hosted copper deposits occurring in sandstones, shales, and marls are enormous in size and their contained resources are comparable to porphyry copper deposits. These were most-likely formed diagenetically by groundwater fluids in highly-permeable rocks [45]. Well-known examples are the Kupferschiefer in Europe, which has an areal coverage of >500,000 Km2, and the Zambian Copper Belt in Africa.
![Rob Lavinsky, <a rel="nofollow" class="external text" data-cke-saved-href="http://www.irocks.com/" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/3.0" href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AApatite-(CaF)-280343.jpg" href="https://commons.wikimedia.org/wiki/File%3AApatite-(CaF)-280343.jpg">via Wikimedia Commons</a> The crystal is hexagonal and light green.](https://bio.libretexts.org/@api/deki/files/57545/Apatite-CaF-280343-300x267.jpg?revision=1)
Phosphorus is an essential element that occurs in the mineral apatite, which is found in trace amounts in common igneous rocks (Figure \(\PageIndex{15}\)). Phosphorite rock, which is formed in sedimentary environments in the ocean [50], contains abundant apatite and is mined to make fertilizer. Without phosphorus, life as we know it is not possible. Phosphorous is a major component of bone and a key component of DNA. Bone ash and guano are natural sources of phosphorus.
![By Werner Schellmann (Own work) [<a data-cke-saved-href="http://creativecommons.org/licenses/by-sa/2.5" href="http://creativecommons.org/licenses/by-sa/2.5">CC BY-SA 2.5</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3ABauxite_with_unweathered_rock_core._C_021.jpg" href="https://commons.wikimedia.org/wiki/File%3ABauxite_with_unweathered_rock_core._C_021.jpg">via Wikimedia Commons</a> The outside of the rock is tan and weathered, the inside is grey.](https://bio.libretexts.org/@api/deki/files/57548/Bauxite_with_unweathered_rock_core._C_021-300x195.jpg?revision=1&size=bestfit&width=338&height=220)
Residual mineral deposits can form when weathering processes remove water soluble minerals from an area, leaving a concentration of less soluble minerals. The aluminum ore, bauxite, was originally formed in this manner under tropical weathering conditions [46] (Figure \(\PageIndex{16}\)). The best known bauxite deposit in the United States occurs in Arkansas. Aluminum concentrates in soils as feldspar and ferromagnesian minerals in igneous and metamorphic rocks undergo chemical weathering processes. Weathering of ultramafic rocks results in the formation of nickel-rich soils and weathering of magnetite and hematite in banded iron formation results in the formation of goethite, a friable mineral that is easily mined for its iron content.
At the earth's surface, the physical process of mass wasting or fluid movement concentrates high-density minerals by hydraulic sorting. When these minerals are concentrated in streams, rivers, and beaches, they are called placer deposits, whether in modern sands or ancient lithified rocks [47] (Figure \(\PageIndex{17}\)). Native gold, native platinum, zircon, ilmenite, rutile, magnetite, diamonds, and other gemstones can be found in placers. Humans have mimicked this natural process to recover gold manually by gold panning and by mechanized means such as dredging.
![By Photograph taken by Mark A. Wilson (Department of Geology, The College of Wooster). [1] (Original photograph) [Public domain], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AHeavyMineralsBeachSand.jpg" href="https://commons.wikimedia.org/wiki/File%3AHeavyMineralsBeachSand.jpg">via Wikimedia Commons</a> The tan rock has dark streaks of minerals.](https://bio.libretexts.org/@api/deki/files/57546/HeavyMineralsBeachSand-300x205.jpg?revision=1&size=bestfit&width=337&height=230)
The best types of aggregate (sand and gravel) resources are those that have been sorted by streams, and in Canada the most abundant and accessible fluvial deposits are associated with glaciation (Figure \(\PageIndex{18}\)). That doesn't include till of course, because it has too much silt and clay, but it does include glaciofluvial outwash, which is present in thick deposits in many parts of the country, similar to the one shown in Figure 20.15. In a typical gravel pit, these materials are graded on-site according to size and then used in a wide range of applications from constructing huge concrete dams to filling children's sandboxes. Sand is also used to make glass, but for most types of glass, it has to be at least 95% quartz (which the sandy layers shown in Figure 20.15 are definitely not), and for high-purity glass and the silicon wafers used for electronics, the source sand has to be over 98% quartz.

Figure \(\PageIndex{18}\): Sand and gravel in an aggregate pit near Nanaimo, BC. [SE]
Evaporite deposits form in restricted basins, such as the Great Salt Lake or the Dead Sea, where evaporation of water exceeds the recharge of water into the basin [49] (Figure \(\PageIndex{19}\)). As the waters evaporate, soluble minerals are concentrated and become supersaturated, at which point they precipitate from the now highly-saline waters. If these conditions persist for long stretches of time, thick deposits of rock salt and rock gypsum and other minerals can accumulate.
![By Hermann Luyken (Own work) [<a data-cke-saved-href="http://creativecommons.org/publicdomain/zero/1.0/deed.en" href="http://creativecommons.org/publicdomain/zero/1.0/deed.en">CC0</a>], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3A2012.10.02.111543_Bonneville_Salt_Flats_Utah.jpg" href="https://commons.wikimedia.org/wiki/File%3A2012.10.02.111543_Bonneville_Salt_Flats_Utah.jpg">via Wikimedia Commons</a> The ground is white and flat for a long distance.](https://bio.libretexts.org/@api/deki/files/57536/Bonneville_Salt_Flats_Utah-300x200.jpg?revision=1&size=bestfit&width=342&height=228)
Evaporite minerals like halite are used in our food as common table salt. Salt was a vitally important economic resource prior to refrigeration as a food preservative. While still used in food, now it is mainly mined as a chemical agent, water softener, or a de-icer for roads. The largest salt mine in the world is at Goderich, Ontario, where salt is recovered from the 100 m thick Silurian Salina Formation. Gypsum (CaSO4.2H20) is a common nonmetallic mineral used as a building material, being the main component of drywall. It is also used as a fertilizer. Other evaporites include sylvite (potassium chloride) and bischofite (magnesium chloride), both of which are used in agriculture, medicine, food processing, and other applications. Potash, a group of highly soluble potassium-bearing evaporite minerals, is used as a fertilizer. In hyperarid locations, even rarer and more complex evaporites, like borax, trona, ulexite, and hanksite, are found and mined. They can be found in such localities as Searles Dry Lake and Death Valley, California, and in ancient evaporite deposits of the Green River Formation of Utah and Wyoming.
References
30. Boudreau, A. E. The Stillwater Complex, Montana – Overview and the significance of volatiles. Mineralogical Magazine 80 , 585–637 (2016).
31. Willemse, J. The geology of the Bushveld Igneous Complex, the largest repository of magmatic ore deposits in the world. Economic Geology Monograph 4 , 1–22 (1969).
32. London, D. & Kontak, D. J. Granitic Pegmatites: Scientific Wonders and Economic Bonanzas. Elements 8 , 257–261 (2012).
33. Arndt, N. T. Chapter 1 Archean Komatiites. in Developments in Precambrian Geology (ed. K.C. Condie) 11 , 11–44 (Elsevier, 1994).
34. Barrie, C. T. Volcanic — associated massive sulfide deposits: processes and examples in modern and ancient settings. (1999). Available at: https://www.researchgate.net/profile/Michael_Perfit/publication/241276560_Geologic_petrologic_and_geochemical_relationships_between_magmatism_and_massive_sulfide_mineralization_along_the_eastern_Galapagos_Spreading_Center/links/02e7e51c8707bbfe9c000000.pdf. (Accessed: 2nd July 2016)
35. Richards, J. P. Tectono-Magmatic Precursors for Porphyry Cu-(Mo-Au) Deposit Formation. Econ. Geol. 98 , 1515–1533 (2003).
36. Hawley, C. C. A Kennecott Story: Three Mines, Four Men, and One Hundred Years, 1887-1997 . (University of Utah Press, 2014).
37. Ague, J. J. & Brimhall, G. H. Geochemical modeling of steady state fluid flow and chemical reaction during supergene enrichment of porphyry copper deposits. Econ. Geol. 84 , 506–528 (1989).
38. Einaudi, M. T. & Burt, D. M. Introduction; terminology, classification, and composition of skarn deposits. Econ. Geol. 77 , 745–754 (1982).
39. Bromfield, C. S., Erickson, A. J., Haddadin, M. A. & Mehnert, H. H. Potassium-argon ages of intrusion, extrusion, and associated ore deposits, Park City mining district, Utah. Econ. Geol. 72 , 837–848 (1977).
40. James, L. P. Geology, ore deposits, and history of the Big Cottonwood Mining District, Salt Lake County, Utah . (Utah Geological and Mineral Survey, Utah Department of Natural Resources, 1979).
41. Hofstra, A. H. & Cline, J. S. Characteristics and models for Carlin-type gold deposits. Reviews in Economic Geology 13 , 163–220 (2000).
42. Rui-Zhong, H., Wen-Chao, S., Xian-Wu, B., Guang-Zhi, T. & Hofstra, A. H. Geology and geochemistry of Carlin-type gold deposits in China. Miner. Deposita 37 , 378–392 (2002).
43. Lehmann, I. P', Publ. Bur. Centr. Seism. Internat. Serie A 14 , 87–115 (1936).
44. Klein, C. Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins. Am. Mineral. 90 , 1473–1499 (2005).
45. Hitzman, M., Kirkham, R., Broughton, D., Thorson, J. & Selley, D. The Sediment-Hosted Stratiform Copper Ore System. Econ. Geol. 100th , (2005).
46. Bárdossy, G. & Aleva, G. J. J. Lateritic bauxites . 27 , (Elsevier Science Ltd, 1990).
47. Cabri, L. J., Harris, D. C. & Weiser, T. W. Mineralogy and distribution of platinum-group mineral (PGM) placer deposits of the world. Explor. Min. Geol. 2 , 73–167 (1996).
48. Nuss, P. & Eckelman, M. J. Life cycle assessment of metals: a scientific synthesis. PLoS One 9 , e101298 (2014).
49. Gordon, W. A. Distribution by latitude of Phanerozoic evaporite deposits. J. Geol. 83 , 671–684 (1975).
50. Delaney, M. L. Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle. Global Biogeochem. Cycles 12 , 563–572 (1998).
Source: https://bio.libretexts.org/Courses/University_of_Pittsburgh/Environmental_Science_(Whittinghill)/06%3A_Geology/6.05%3A_Geologic_Resources/6.5.01%3A_Mineral_Resources

0 Response to "Easy Mineral Resource is There Lead Mining in Minnesota"
Post a Comment